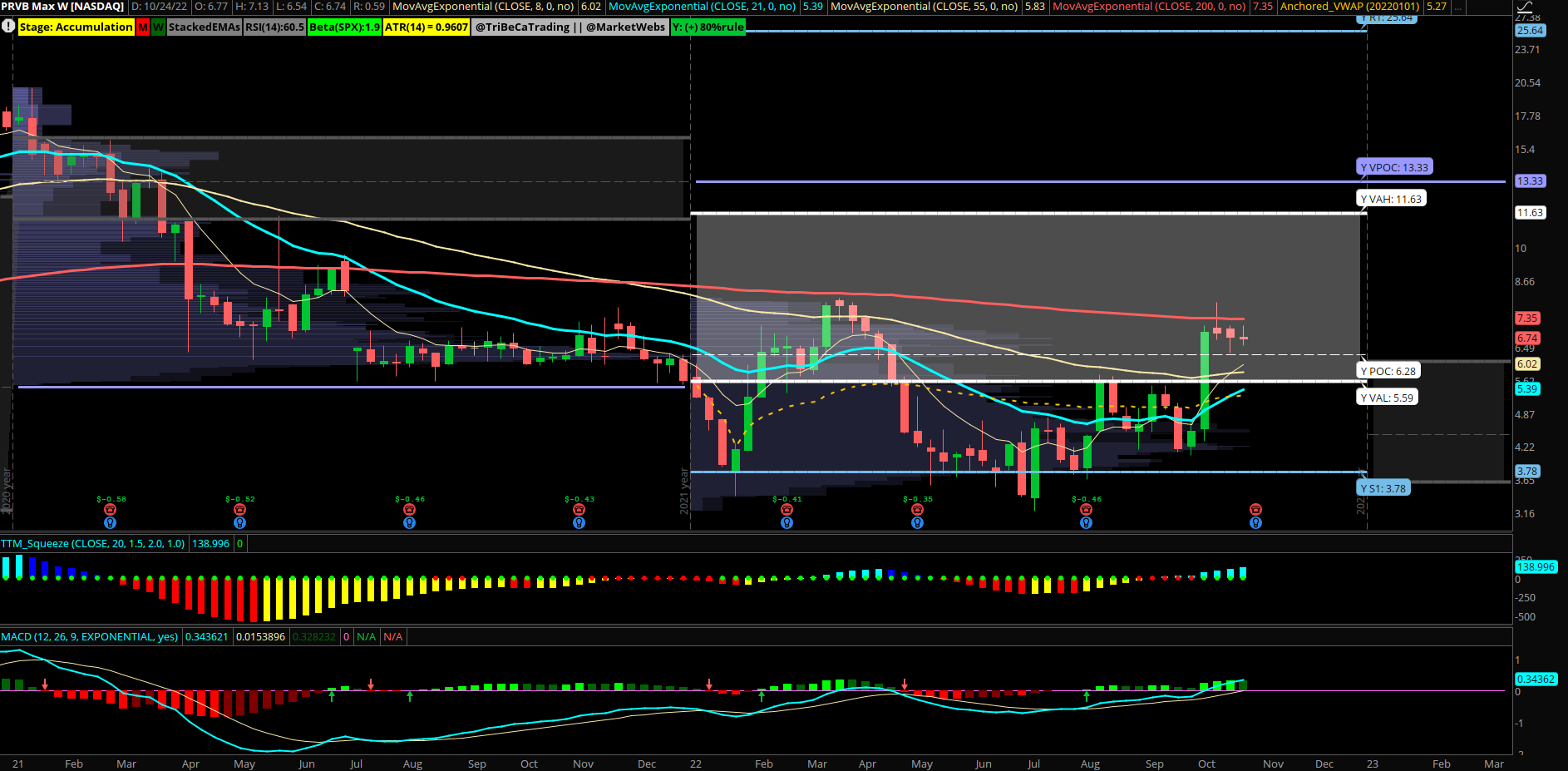
Small Biotech Name Sees Unusual Call Fly Buyer
Provention Bio (PRVB) small bio name opens 10,000×20,000x November $7.50/$10/$12.50 call fly’s for $0.49 debit, earnings 11/3. Unusual sized trade for the small bio as the premium paid was $490k in total and looks to be targeting a move higher towards 10 possibly into next month. The stock broke above key resistance at 5.60 recently and forming a bull flag now on top of yearly VPOC support.
A $550M biotech that focuses on intercepting and preventing immune-mediated diseases. The Company’s product pipeline includes PRV-031 (teplizumab), PRV-3279, PRV-015 (ordesekimab) and PRV-101. Teplizumab is a humanized, anti-CD3 monoclonal antibody (mAb) for the delay of clinical type 1 diabetes (T1D) in at-risk individuals and for patients with newly diagnosed T1D. PRV-3279 is a humanized bispecific scaffold molecule targeting the B-cell surface proteins, CD32B and CD79B, for the treatment of systemic lupus erythematosus (SLE) and for the prevention of immunogenicity of biotherapeutics, such as those used in gene therapy. Ordesekimab is a human anti-interleukin 15 (IL-15) mAb for the treatment of gluten-free diet non-responsive celiac disease (NRCD). PRV-101 is a coxsackievirus B (CVB) vaccine to prevent acute CVB infections and, in those patients at-risk, to prevent the CVB-triggered autoimmune damage to pancreatic beta-cells.
On 10/6, Provention Bio (PRVB) entered into a co-promotion agreement with Sanofi U.S. (SNY) for the launch of Provention’s lead investigational drug candidate teplizumab. The agreement enables Provention Bio to leverage Sanofi’s expertise, capabilities and commercial resources to support the potential launch of teplizumab currently under review by the FDA, for the delay of clinical type 1 diabetes in at-risk individuals, with a user fee goal date of November 17, 2022 for the Biologics License Application. The Company also granted Sanofi, in consideration of a one-time payment of $20 million, an exclusive, one-time right of first negotiation (ROFN) to obtain exclusive global rights to commercialize teplizumab for Type 1 diabetes indications in humans, subject to certain retained rights of the Company to engage in discussions with third parties with respect to certain transactions. Sanofi may exercise the ROFN, until June 30, 2023, with an option to extend within 2023 under certain conditions. Pursuant to the Purchase Agreement, if teplizumab is approved by the FDA, Sanofi has agreed to purchase $35 million of the Company’s common stock at a premium over the daily volume-weighted average per share price for the five consecutive trading days prior to the closing date.